 RESTORE aims to develop a smart nanobiomaterial-based solution capable to modulate the articular cartilage microenvironment from a state of limited capacity of repair towards one that enables true regeneration of both tissue structure and function. One approach is the development and validation of a nanoenabled COPLA® scaffold for cartilage repair.
RESTORE aims to develop a smart nanobiomaterial-based solution capable to modulate the articular cartilage microenvironment from a state of limited capacity of repair towards one that enables true regeneration of both tissue structure and function. One approach is the development and validation of a nanoenabled COPLA® scaffold for cartilage repair.
The ability of the nanoenabled COPLA® scaffold to enhance cartilage defect regeneration is assessed in a rabbit osteochondral lesion model. The rabbit represents a commonly used model for early investigations of cartilage defect repair strategies. Main advantages include the long-term experience with these animals in biomedical research and their larger joints compared to rodents, facilitating surgical procedures and the creation of relevant cartilage defects. Furthermore, the availability of larger tissue samples allows for comprehensive biomechanical and histological analyses.
The animal study was approved by the Local Ethical Committee and is conducted according to the national and international regulations for the care and use of laboratory animals considering the 3Rs principle (Replacement, Reduction, Refinement).
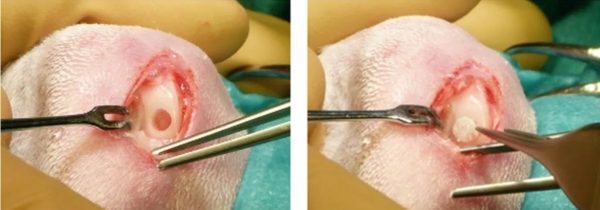
For in vivo evaluation, the scaffold is implanted into a critical sized osteochondral defect (diameter = 3 mm, depth = 2 mm) on the load-bearing area of the medial femoral condyle. Location and diameter of the defect were chosen according to recommendations of the ASTM, reporting that full-thickness cartilage defects larger than 3 mm will not regenerate spontaneously without any intervention (ASTM (F2451-05 (2010)). Two time points (4 and 12 weeks) are evaluated, to assess early and late cell responses. The animal study is ongoing, first results are expected in the upcoming months.
in vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage
Reference: ASTM F2451-05(2010), Standard Guide for in vivo Assessment of Implantable Devices Intended to Repair or Regenerate Articular Cartilage (Withdrawn 2019), ASTM International, West Conshohocken, PA, 2010
www.brinter.com