 AIM Standard 7351731 Offers Guidance Evaluating the Immunity of Medical Devices from Exposure to RFID
AIM Standard 7351731 Offers Guidance Evaluating the Immunity of Medical Devices from Exposure to RFID
AIM, the trusted worldwide industry association for the automatic identification industry, providing unbiased information, educational resources and standards to providers and users of these technologies for nearly half a century, announced recently the release today of Medical Electrical Equipment & System Electromagnetic Immunity Test for Exposure to RFID Readers Revision 3.00, a revised standard that provides specialized guidance on the testing of non-implantable medical devices to determine if they are immune to emissions from radio frequency identification (RFID) systems.
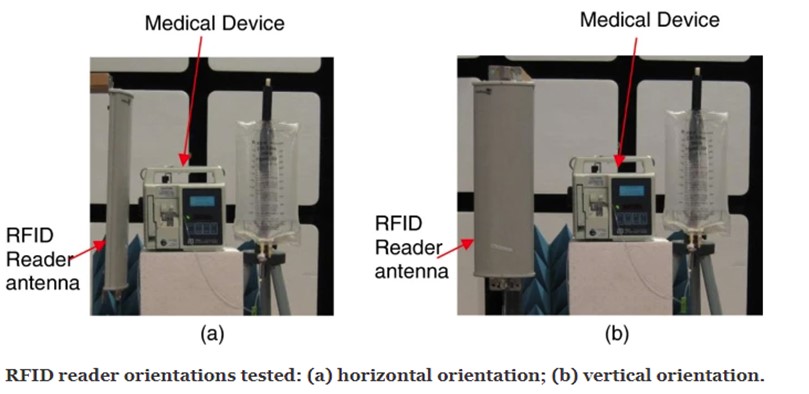
The standard provides medical device manufacturers and end-users with guidance on how to evaluate their devices for immunity to emissions from RFID systems. The test procedures in this document are based on experimental results from several AIM members. Test protocols are included for the major commercial implementations of RFID as standardized by ISO, including LF, HF, and UHF RFID. Both active and passive ISO RFID standards are covered in this document.
Members of AIM’s RFID Experts Group Healthcare Initiative Work Group (REG HCI) developed two prior editions of this standard, and this edition was developed after taking into consideration the comments received on those editions and updates to other publications.
Amendment 1 to IEC 60601-1-2:2014 was published in September 2020 as IEC 60601-1-2:2020, consolidated Edition 4.1. This edition of IEC 60601-1-2 adds proximity magnetic field immunity tests at the RFID frequencies 132.4 kHz, at 65 A/m, and 13.56 MHz, at 7.5 A/m, with the applicability determined by risk analysis. The modulation is specified as pulse modulation, 50% duty cycle, at 2.1 kHz for 134.2 kHz and 50 kHz for 13.56 MHz. The specified test method is IEC 61000-4-39.
Jukka Voutilainen, CEO and co-founder of Voyantic Ltd., helped led the effort within the REG HCI to revise the document, shared this upon the revised standard’s publication. "The AIM standard has an important role in ensuring that it is safe to deploy RFID readers near medical equipment. Several test labs have been testing against the standard for years and have provided AIM with suggestions for improvement. After careful consideration, the group has been able to finish the third standard version that aims to both reflect the latest testing needs and be more straightforward to implement.”
www.aimglobal.org